Rationale
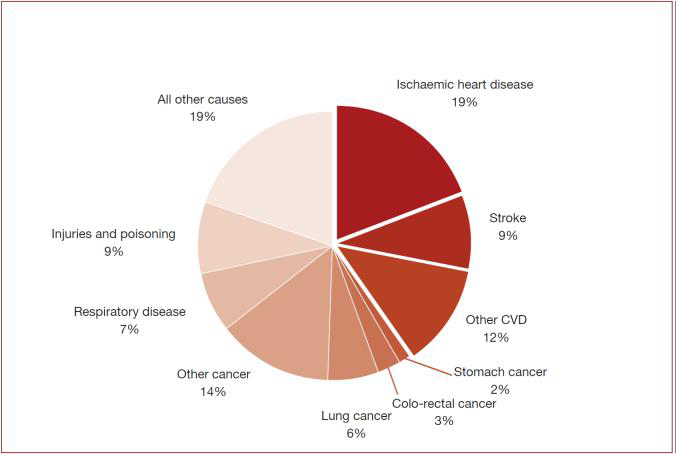
Cardiovascular diseases (CVDs) are disorders of the heart and blood vessels and include coronary heart disease, cerebrovascular disease, valvular disease and other conditions. CVDs take the lives of 17.9 million people every year, 31% of all global deaths.
Today, CVD diagnosis and risk stratification mainly focus on identifying high-risk individuals, by means of “classical” risk factors such as age, blood pressure, lifestyle parameters (e.g. smoking) blood glucose and cholesterol. Patients identified to be at high risk are taken in care and offered interventions on modifiable risk factors. In case of orienting symptoms such as chest pain, breathlessness and neurological deficit, they are diagnosed in specialized medical centers by ultrasound (cardiac echo and/or echographic examination of superficial vessels in the neck region), CT angiogram (CTA) of the head and neck and/or heart region, or magnetic resonance angiography (MRA) and then follow specific care. It is common opinion in the medical community that none of the diagnostic methods mentioned above can be used to optimally diagnose a substantial part of the population at risk starting from the primary care but also the secondary prevention, and that those individuals that are considered to be at low or moderate risk might be undiagnosed because risk factors are silent.
As such, there is a huge interest in deploying the optimal diagnosis to institute a timely preventative therapy among those individuals considered to be at low or moderate risk according to current guidelines.
Three important CVD risk markers are:
- Carotid-femoral PWV as a measure for arterial stiffness
- Arterial stenosis (narrowing of the artery by inflammatory build-up of atheromatous plaque).
- Dyssynchrony or abnormal cardiac contraction patterns

Arterial stiffness
Over the past 10 years, a large amount of evidence has accumulated demonstrating that increased arterial stiffness is an important risk factor for cardiovascular disease especially in its close relationship with hypertension. Hypertension, also known as high blood pressure, is a chronic medical condition in which the blood pressure in the arteries is persistently elevated. Aortic stiffness can be assessed in a number of ways, but the non-invasive measurement of the carotid- femoral (aortic) pulse wave velocity (aPWV) is regarded as the current reference technique. Given the importance of arterial stiffness assessment in relation with hypertension, the assessment of arterial stiffness by measurement of aPWV is included in the latest European Society of Hypertension/European Society of Cardiology guidelines. The Societies suggest that an aPWV of 10 m/s can be considered as the threshold of an estimate of subclinical organ damage in hypertensive patients and individuals with carotid-femoral PWV higher than this threshold should seek clinical treatments to prevent cardiovascular events.
Arterial stenosis
The primary point of care method to assess carotid stenosis is auscultating the carotid artery with a stethoscope. In the case of a stenosis, a swishing noise called a “bruit” might be observed, which may be a sign of turbulent blood flow caused by atherosclerosis. Carotid stenosis can only be confirmed by a carotid ultrasound scan, which has a huge cost if proposed to all patients at risk.
Dyssynchrony
In the primary point-of-care unit, cardiac dysfunction may be detected primarily based on clinical symptoms (chest pain, exhaustion) and auscultation (e.g. suspicious murmurs due to abnormal blood flow patterns). There are no tools and devices available for a reliable, fast, relatively inexpensive and non-invasive detection of heart failure in the primary point-of-care unit. The closest approximation to this would be echocardiography.
All of the above-mentioned CVD risk markers may be obtained from the mechanical movements of the skin induced by the cardiac action and resulting arterial pulses at different parts of the body, e.g. the carotid-femoral PWV derived from the pulse transit time between the femoral and carotid arteries, vibrations resulting from turbulence in a stenosed artery and vibrations induced by the contraction pattern of the heart.
The InSiDe demonstrator device
InSiDe targets to develop a multi-beam LDV device to conduct an easy measurement of the mechanical movements generated by pulses. The device is similar to the device demonstrated in the CARDIS project (hyperlink to www.cardis-h2020.eu). An LDV device is an optical interferometer that measures the phase/frequency change of laser light reflected from a target. If the target is moving, especially vibrating, the phase/frequency of the reflection beam will vary as a result of the Doppler effect.
The InSiDe demonstrator device will be designed to record timed traces of mechanical displacement and vibrations of the skin based on laser doppler vibrometry (LDV), i.e. detect vibrations down in the nanometer range from 1310nm laser light reflected from the skin.
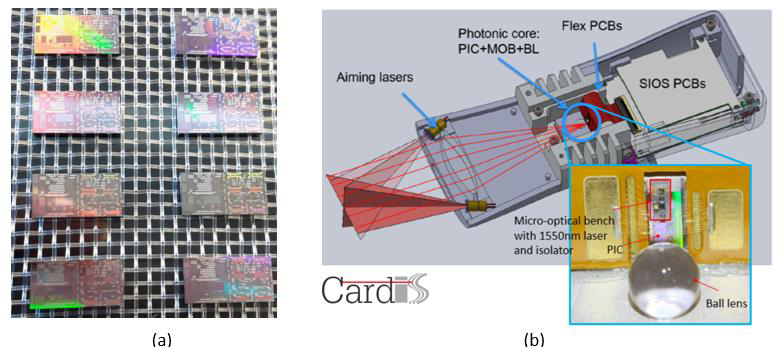
This phase change, which represents the displacement can be retrieved by several techniques. The InSiDe demonstrator device will use a 4-beam homodyne interferometer, in which the reflection beam is combined with a reference beam from the same laser source in a 90-degree optical hybrid and the superposed beams are detected by four photodetectors (PDs). A DSP-based demodulator will retrieve the phase of the reflected beam using the four photocurrents. The multi-beam interferometer, except from the laser source and focusing lens, will be realized in a silicon chip. Thanks to the use of CMOS technology, silicon photonic integrated circuits (PICs) have the potential to be low cost for volume production. The laser source will be attached to the PIC by using a micro-optical bench (MOB). The MOB will be aligned to the input grating coupler of the PIC and glue-fixated. The photonics assembly with the PIC and the MOB will be connected to a PCB responsible for signal conditioning and signal processing.
Wireless hand-held LDV device
The InSiDe project will generate a battery-operated, wireless, hand-held split-device with an integrated spacer and a touch-screen to provide a real-time feedback of the signal quality to the user. It includes two hand-pieces, each having 4 sensing beams (to avoid alignment difficulties) that can be used as a combined device for local stenosis or cardiac contraction measurements or as two separated units for synchronized pulse wave measurements to derive carotid-femoral pulse wave velocity. The envisioned device is shown below.

It includes a master handpiece with the touch screen and three trigger buttons to improve the usability, and a slave handpiece that only does the measurement and transfers the data wirelessly to the master hand piece
The main components of the main handpiece are shown below (a). The slave handpiece will be similar but without the touch screen and the DSP module 2. The sensing part of the hand-piece is a hermetically sealed butterfly-type photonic package comprising of the PIC and the MOB, with a proper ESD protection (b). The electronics used for signal conditioning, ADC and DSP demodulation will also be integrated in the hand-held package, meaning both the raw signal demodulation and the high-level signal-processing, e.g. PWV calculation, will be done in the handpiece.

Clinical feasibility studies for therapeutic diagnosis
Several new LDV-based applications in the CVD field have been introduced as the result of the exploitation of CARDIS. InSiDe will expand the clinical feasibility studies to these new applications, i.e. cardiac diagnosis, carotid stenosis, resistance hypertension and renal denervation, valvular diseases, and barostimulation. In these studies, the LDV device will be used for therapeutic diagnosis rather than screening.
Validated clinical algorithms
The InSiDe device will provide a real-time feedback to the operator to indicate the measurement signal quality and even to tell the operator how to improve the measurement locations. Secondly, InSiDe will develop the specific clinical algorithms for different applications, especially for carotid-femoral PWV, cardiac dyssynchrony and stenosis detection. The algorithms will be validated by comparing with standard reference techniques in the clinical feasibility studies.
Readiness for volume manufacturing
InSiDe will enable an industrialization study and make the LDV device ready for high-volume manufacturing. Costs of MOB, packaging and electronics will be considerably reduced by design for manufacture, e.g. the price of the MOB integration can be reduced by using automatic integration processes and a wafer-level processed MOB platform. Problems of different parts, e.g. PIC design, will be checked and possibly redesigned to aid volume manufacture. An industrial supply chain will also be initiated in InSiDe. As a summary, in InSiDe we will design and fabricate a wireless, hand-held, volume-production-ready multibeam LDV device for therapeutic monitoring of various CVD problems, with effective clinical algorithms. The device needs no retro-reflective patches to enhance reflection. The outputs of the device will be validated by intensive clinical feasibility studies in various CVD applications.
Positioning of the project
The InSiDe project plan the release of a clinical investigational device (medical grade device with restricted use) to be tested in clinical practice to diagnose a disease stage and timely initiate a specific therapy or possibly change the course of an ongoing one. The InSiDe project aims to develop a prototype device very close to a first commercial product, developed to a stage where production and assembly processes are scalable to large volume production.
Related national and international research and innovation activitiesThe InSiDe project builds upon results from the H2020 CARDIS project on ‘Early-stage cardiovascular disease detection with integrated silicon photonics’. (link to www.cardis-h2020.eu)